PRODUCT FAQS
Search by topics:
GENERAL INTERFERENCE TESTING QUESTIONS
Q: Are there regulations that require me to perform interference testing in my laboratory?
Q: Are there guidelines for performing interference testing experiments, including analyte concentrations?
Q: What are some of the most common interferents?
A: Interference from hemolysis (hemoglobin and /or red blood cell contents), lipemia (triglyceride-rich lipoproteins and turbidity), icterus (unconjugated or conjugated bilirubin), and proteins (albumin and gamma-globulins, paraproteins) are so common with routine chemistry assays, that validation by manufacturers always includes interference testing for these substances.
Q: What type of material is used to perform interference testing?
A: The EP7-A guidance document outlines materials that can be used to perform interference testing. However, many of the materials are difficult to obtain. Additionally, labs may store abnormal patient samples that fit their needs or use artificial interferent substitutes. For example, lipema is often simulated using IntraLipid®. Unfortunately, the photometric response to this synthetic “fat” differs from physiological lipema and is inappropriate for interference studies.
ASSURANCE™ QUESTIONS
Precision products for empirical results.

Q: What interferents are included in the ASSURANCE™ Interference Test Kit INT-01?
- Human triglyceride-rich lipoproteins for evaluation of lipemia interference;
- Human hemolysate for evaluation of hemolysis interference;
- A mixture of human serum albumin and gamma-globulins to evaluate protein interference;
- Conjugated and unconjugated bilirubin to evaluate interference from icterus.
Q: What preservatives are added to the materials?
Q: How will ASSURANCE benefit my laboratory?
Q: How do I perform interference testing?
A: The Clinical and Laboratory Standards Institute (CLSI) has approved a guidance document on interference testing: CLSI. Interference Testing in Clinical Chemistry; 3rd ed. CLSI guideline EP7. Clinical and Laboratory Standards Institute, Wayne, PA, 2018. Sun Diagnostics has created a recommended testing protocol based on CLSI EP7-A guidelines. This protocol is available for download here.
Q: How do I analyze and interpret the data generated from my interference studies?
A: Sun Diagnostics has created a data analysis tool based on CLSI EP7-A guidelines.
Q: How do I know the actual concentrations of the interferents in the kit?
A: As discussed in the testing protocol, we recommend that you assay the materials prior to beginning your experiments. This will provide information on how the materials are responding on your specific analyzer. If it isn’t feasible to assay the materials, you can use the “typical values” provided in the package insert as a guide. For more information, contact Sun Diagnostics Tech Support via email support@sundiagnostics.us or via phone 1-877-SUN-DIAG (877-786-3424)
GENERAL INTERFERENCE TESTING QUESTIONS
Q: What are the traditional approaches to precipitating lipoproteins? What are their drawbacks?
Q: Is there another way to precipitate lipoproteins?
LIPOSEP IP QUESTIONS
Q: What are some benefits to LipoSep IP™?
- Is a highly specific tool to isolate HDL in serum, allowing for HDL-C measurement using a Total Cholesterol assay. TCHOL assays are less expensive, more standardized, and more accurate than homogeneous HDL-C assays.
- Has a simple, straightforward procedure
Does not alter lipoprotein particle composition - Other HDL components, such as apo AI, apo E, and myeloperoxidase can also be measured.
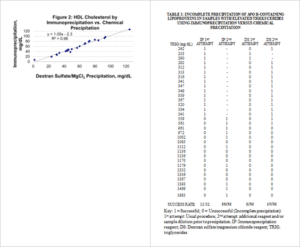
Q: How does immunoprecipitaiton with LipoSep IP compare to chemical precipitation with dextran sulfate?
We want to work with you.
(207) 926-1125
Sun Diagnostics LLC
60 Pineland Drive Brunswick Hall
Suite 322
New Gloucester, ME 04260