Reports of biotin interference with laboratory tests are alarming. Biotin binds to streptavidin with high affinity, making the biotin-streptavidin system useful for immunoassays; however, elevated concentrations of biotin can interfere.
In competitive assays, useful for small molecules such as thyroxine (T4), there is an inverse association between analyte concentration and assay signal; biotin interference causes a low signal and a falsely high result. In sandwich assays, like a thyroid-stimulating hormone (TSH), the assay signal is proportional to the analyte concentration, and biotin interference causes a low signal and a falsely low result. High biotin nutritional supplements have been promoted for skin and hair benefits and for conditions such as diabetes and improvement of quality of life in multiple sclerosis. High serum biotin concentrations were noted in individuals taking pharmacological doses of 20 mg per day with some multiple sclerosis patients taking 300 mg per day, resulting in serum biotin concentrations up to 1200 ng/mL. One report found that daily biotin supplementation of only 10 mg resulted in interference with several immunoassays and potential misdiagnosis of thyroid disorders and congestive heart failure.
Consequently, the FDA released a safety communication in November 2017 and a draft guidance document for manufacturers in June 2019. The AACC has also recently released guidelines (January 2020).
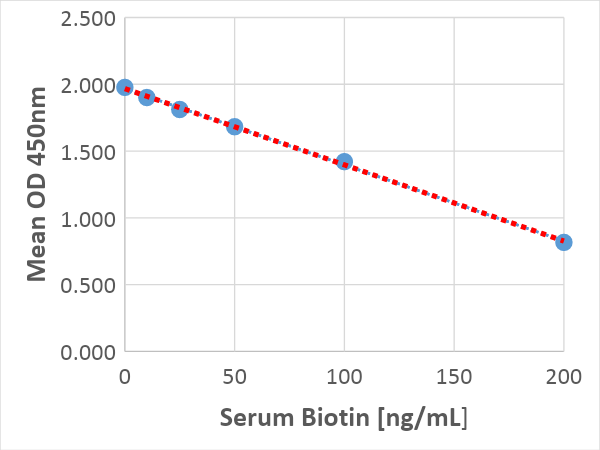
In response to the need for tools to assist with the validation and investigation of biotin as a possible interferent, Sun Diagnostics has developed a rapid microtiter plate assay to measure potentially interfering concentrations in patient samples. The biotin assay is available as a research-use only (RUO) kit and takes only about 75 minutes. The interference test has a limit of blank (LoB) of 13 ng/mL, a limit of detection (LoD) of 32 ng/mL and is linear up to 200 ng/mL. Total imprecision ranged from 4.2% at 35 ng/mL to 13.2% at 175 ng/mL. Our biotin assay can be used to confirm elevated biotin a source of interference, but it is not sensitive enough to measure normally low biotin concentrations.
Sun Diagnostics also provides concentrated biotin material for interference tests and interference experiments. The material is available in 1 mL aliquots at 20-fold the recommended test concentration of 3510 ng/mL (70,200 ng/mL), allowing for use at a 1:20 dilution in the sample matrix.
We are also developing a biotin clearing reagent and a simple procedure to remove biotin interference. The specimen is mixed with NeutrAvidin, a deglycosylated version of avidin, in a microcentrifuge tube with a filter insert, vortexed, and centrifuged. Biotin binds strongly to the NeutrAvidin-coated beads and is retained in the filter. The filtrate, collected at the bottom of the tube, is suitable for analyses.
We are hopeful that these new tools will be useful to laboratorians to better understand biotin interference with their laboratory tests and allow them to better protect against the reporting of false-positive and false-negative results.